Lab-Grown Rat Limb By Massachusetts General Hospital Paves Way For Bioengineered Human Transplants
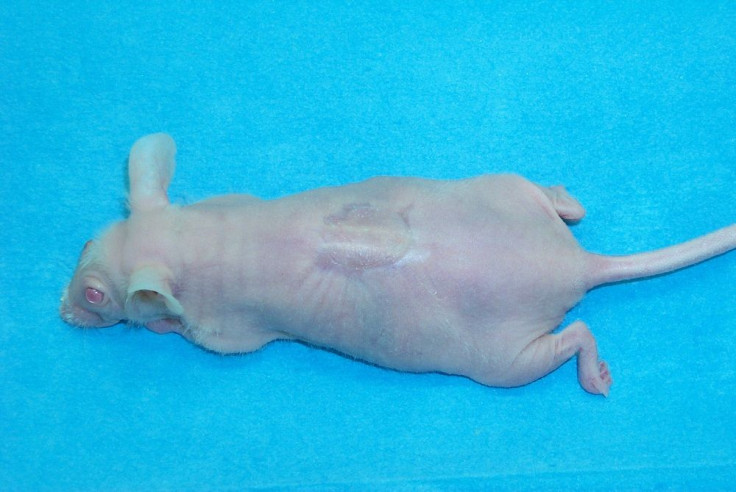
Researchers at Massachusetts General Hospital has successfully grown in the laboratory a rat limb. The rodent limb has functioning veins and muscle tissue. The result of the research was published in this week’s issue of Biomaterials journal.
The scientists took forelimbs from a dead rat, soaked it in a detergent solution to remove all cellular materials, which preserved the primary vascular and nerve matrix. The rest of the materials was used to form the new cellular material’s structural basis. The team then placed the stripped limb matrix in a container called bioreactor and then injected the vein cells into the main artery of the limbs. Muscle progenitor cells were also injected into the matrix sheaths. To grow the cells, the bioreactor was filled with a nutrient solution. The researchers applied electrical stimulation after five days to promote the formation of muscles.
The success of the experiment makes transplant surgery of human limbs possible by using biological material from the patient, reports CNET. The muscles and veins were grown from stem cells in the laboratory, while the veins were created from the patient’s own cells. By using a patient’s own genetic materials, chances of rejection after a transplant is lower and it takes away the need for the patient to take immunosuppressant drugs for life.
Dr Harald Ott, the senior author of the research and from the hospital’s Department of Surgery and Center for Regenerative Medicine, points out that the composite nature of limbs makes creating a functional biological replacement challenging. The procedure requires rebuilding of each muscles, bones, cartilages, blood vessels, tendons, ligaments and nerves and the matrix, its specific supporting structure.
“We have shown that we can maintain the matrix of all these tissue in their natural relationships to each other, that we can culture the entire construct over prolonged periods of time, and that we can repopulate the vascular system and musculature,” Ott said. He adds that because a limb has more than a single tissue type, growing it is a more complex procedure that his team solved by stripping the donor organs of its cells to create a neutral matrix.
A bio-engineered limb, created through the process described above, offers more advantage than robotic prosthetics that need brain training to control the new limb. The former also has the ability to gauge pressure and heat.
Ott said the team would eventually replicate what they did with the rat limb to regenerating muscles using human cells and going further into other tissue types like the bone, cartilage and connective tissue. He said that despite the team’s focus on the hand and forearm as model system and proof of principle, the technique also applies to legs, arms and other body extremities, reports New Science.
To contact the writer, email: vittoriohernandez@yahoo.com





















