First Human Clinical Trial For Experimental Drug Zmapp For Ebola Begins In the U.S. And Liberia
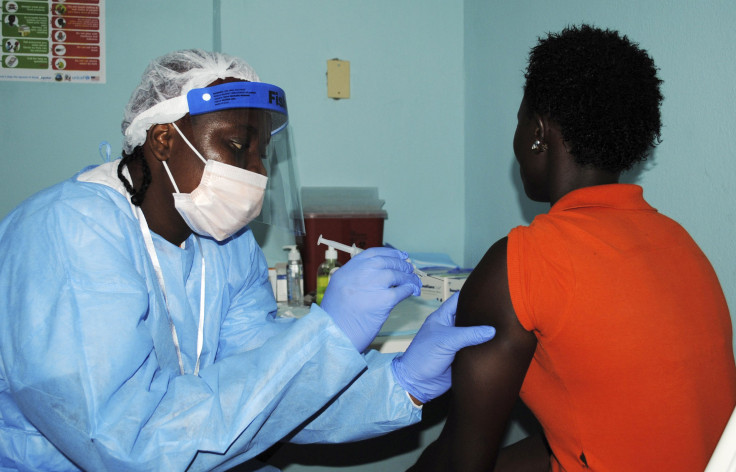
The National Institute of Allergy and Infectious Diseases (NIAID) and the government of Liberia have joined hands to kick-start the clinical trial for Zmapp, the drug which was used in 2014 to treat the infected patients in the Europe, Africa and the United States, under emergency authorisation. The drug by the San Diego-based company, Mapp biopharmaceutical, is being clinically tested for the first time.
“This clinical trial will help us determine if ZMapp and other treatments are safe and effective for use in the current devastating outbreak in West Africa as well as in future outbreaks,” said the director of NIAID, Anthony S. Faudi, in a statement released by the National Institute of Health (NIH).
The clinical testing is being performed on the Ebola-infected individuals admitted in the treatment units in Liberia and the ones in the United States who have acquired the infection through secondary transmission, including the healthcare workers who returned to the U.S. for Ebola treatment.
Under the treatment programme, the infected individuals are injected with intravenous fluids, while the blood oxygen level and blood pressure are monitored and maintained. The management of infection in the human body is carried throughout the treatment programme. To check the efficiency of the drug, a few participants, apart from the standard control group, will receive additional three doses of drugs at an interval of three days. The discharged patients will be followed up after a month to monitor the infection levels.
Zmapp is known to target the surface protein of the Ebola virus, thus stopping its multiplication in the human body. The drug is composed of three different monoclonal antibodies. According to the researchers, the first phase of the trials is expected to end in December 2016. If Zmapp proves to be effective in the trial, then it might be declared as a part of the standard treatment for Ebola-infected patients.
To report a problem or to leave a feedback on the article, send an e-mail to emailtoguneet@gmail.com.