Medtronic gets FDA approval of artificial pancreas which will be available in the market in spring 2017
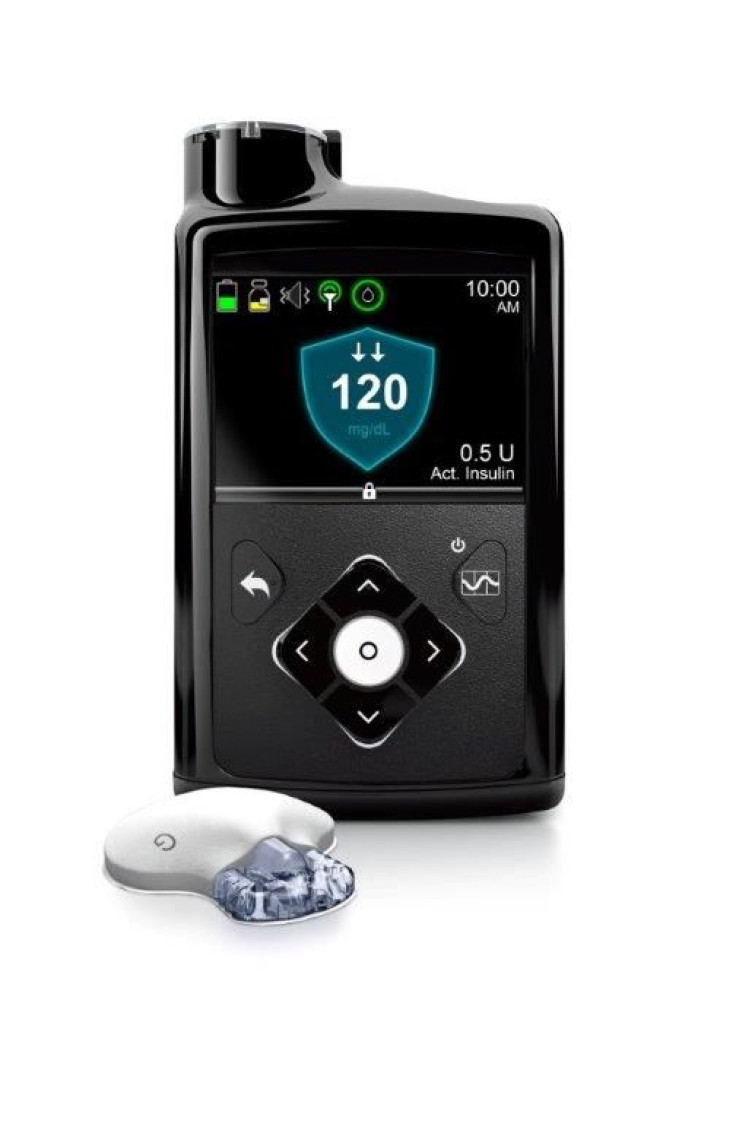
There would be a better way of treating diabetes beginning spring of 2017 with the availability in the market of the world’s first artificial pancreas. The device would be manufactured by medical device maker Medtronic which got on Wednesday approval from the US Food and Drug Administration.
The approved gadget, the MiniMed 670G, is a hybrid closed loop system that automates monitoring of a type 1 diabetic’s blood sugar level and adjusting insulin levels. To perform those functions, the artificial pancreas would have a glucose sensor, insulin pump and the SmartGuard HCL program which is the most advanced algorithm to date, says Medtronic, reports Fortune.
Dr Richard Bergenstal, principal investigator of the study and executive director of the Park Nicollet International Diabetes Center in Minneapolis, explains, “With SmartGuard HCL, the ability to automate basal insulin dosing 24 hours a day is a much-anticipated advancement in the diabetes community for the profound impact it may have on managing diabetes - particularly for minimizing glucose variability and maximizing time in the target range.”
The SmartGuard HCL delivers a variable rate of insulin 24 hours a day based on a patient’s personalised needs, in the process, maximising the time glucose levels are within the target range. The program was designed to learn the patient’s insulin needs and to take action to minimise both high and low glucose levels.
The artificial pancreas was approved for type 1 diabetics 14 years of age and older patients. Medtronic says there are ongoing studies to widen the indication to additional patient populations. But it is not fully automated yet since the patient still needs to know the carbohydrates in their food and enter that information into the system, notes WebMD.
VIDEO: FDA Approves First ‘Artificial Pancreas’ for Diabetes
Source: Sequence Media Group