Massachusetts researchers grow functional heart tissues from stem cells
First, it was various eye cells that researchers grew from stem cells. Now, it is heart tissues that scientists are growing out of skin cells, paving the way, one day, for new hearts to be made for cardiovascular patients needing new ones.
That would mean heart transplant patients would no longer need to wait for a donor match. Instead, a heart could be grown from their stem cells, lowering the chance of the body rejecting the transplanted organ. CNET reports that Massachusetts General Hospital researchers have successfully grown functional heart tissue from stem cells using skin cells.
However, for now it is the heart tissue that could be grown because growing an entire heart from cells is not possible yet since the organ would require a scaffold to give the cells a shape. That scaffold, an extracellular matrix, is created by the cells from proteins secreted.
Besides the structural scaffold, it would also need a supply of specialised cardiac cells and a supportive environment where cells can repopulate the scaffold to form mature tissue capable of handling complex cardiac functions, says Jacques Guyette, lead author of the study published in Circulation Research journal.
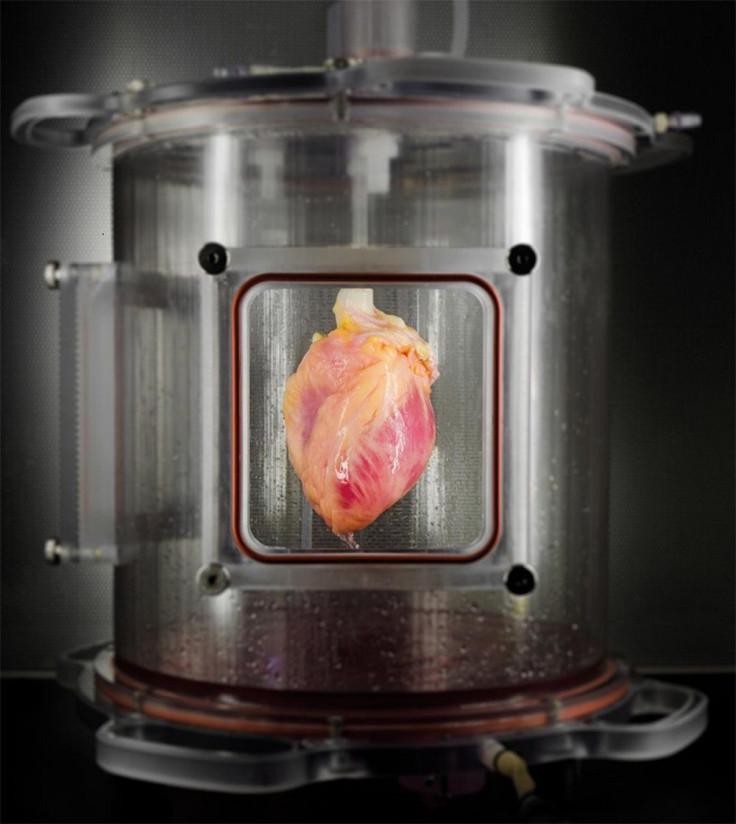
Growing the extracellular matrices takes time, so the Massachusetts team instead used 73 donor hearts from New England Organ Bank which were determined unsuitable for transplantation. The team than stripped it down to the extracellular matrix with a detergent solution, removing all living cells and leaving behind a neutral scaffold for the new cellular material.
The team utilised a new method that uses messenger RNA to seed the matrices with cells to revert the skin cells to stem cells. It is a more efficient technique than the older genetic manipulation method, allowing the pluripotent stem cells to be induced to grow into cardiac muscle cells.
Finally, the muscle cells were introduced into the extracellular matrix which, within days, grew into contracting muscle tissue. The growing heart was then placed in a bioreactor with a nutrient solution and stressors that reproduces the conditions under which a living heart operates. The team found within 14 days dense regions of immature cardiac muscle tissues that contract normally under electrical stimulation.
For now, the functional myocardial tissue is a theoretical alternative to allotransportation, with the patient-derived bioartificial myocardium providing functional support. It is projected to ultimately impact the treatment of heart failure, estimated as affecting 25 million individuals worldwide yearly. In the US alone, there are about 4,000 patients waiting for heart transplants, but there is donor organ shortage with only about 2,500 heart transplants taking place in a year, according to the study.






