Ebola Vaccine Shows High Immune System Response In Early Tests; Australia Lifts Travel Ban on Congo
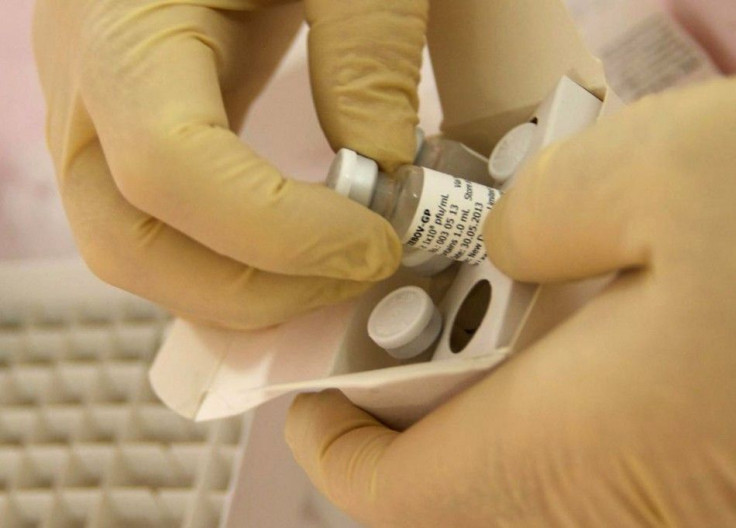
An early test of an experimental Ebola vaccine appears to be safe. Researchers at the National Institutes of Health revealed the results of an early test and gave the go signal for a clinical study in West Africa later in 2014 or in 2015.
The results of the first phase of the Ebola vaccine testing were reported in the New England Journal of Medicine. The report follows a congratulatory statement from the White House which observers have noted as unusual since it was too early to declare victory on Ebola based on initial results, according to the Wall Street Journal.
Scientists developed the vaccine in the NIH Vaccine Research Centre and positive results on safety mirrored the public statements of Anthony Fauci, the director of NIH National Institute of Allergy and Infectious Diseases.
However, challenges still remain before an effective vaccine against Ebola becomes a reality. The vaccine, subjected to early testing, is a collaborative effort between NIH and GlaxoSmithKline PLC. Previous reports have indicated that this particular vaccine is the farthest along in development compared to other experimental drugs on Ebola.
According to the study, the Ebola vaccine was administered in different doses to 20 volunteers divided into two groups. The human volunteers who were given a higher dosage had developed a stronger immune response compared to the other group with smaller doses. The findings can help researchers determine the proper dose in future human trials in West Africa.
Scientists had noted that some volunteers developed a fever within eight to 24 hours after scientists administered the vaccine. The fevers were gone in one day. The vaccine has a chimpanzee cold virus which was genetically modified to hold a non-infectious Ebola protein on the surface.
The World Health Organisation said more than 5,600 people have died from the Ebola outbreak. The latest official death toll comes after case incidence in the two hardest hit countries in West Africa appear to be stabilising. Nearly 16,000 cases have been reported in eight countries that have been touched by Ebola. The WHO has warned that the transmission rate of the Ebola virus in Sierra Leone continues to be "intense."
Meanwhile in Australia, the government has lifted the ban on visitors from Congo. The country is resuming its issuance of entry visas to travellers from Congo since the West African nation was declared Ebola-free on Nov. 15, the NZ Herald notes. Australia has previously suspended the entry visas of travellers from Congo, Liberia, Guinea and Sierra Leone a month ago to prevent Ebola from spreading in the country.