Ebola Outbreak: Vaccine Trials on Humans to Begin Soon, Could Be Marketed By 2015
The World Health Organisation (WHO) has announced over the weekend it will already commence the clinical trials of a preventative vaccine for the Ebola virus next month. The vaccine was made by British pharma company GlaxoSmithKline (GSK). If successful, it could be made available and marketed by 2015.
GSK's drug, co-developed with US scientists, will enter Phase I testing in humans. The only thing delaying its start is the approval from the U.S. Food and Drug Administration.
"We are targeting September for the start of clinical trials, first in the United States and certainly in African countries, since that's where we have the cases," Jean-Marie Okwo Bele, the WHO's head of vaccines and immunisation, told French radio RFI.
He said the results of the clinical trials could come out by end of the year.
He also noted emergency procedures can be put in place so that the vaccine can immediately be manufactured and distributed by 2015.
Marie-Paule Kieny, assistant director-general of the UN health agency, however, told AFP the rush to address the deepening Ebola outbreak in the African region would mean the Ebola cure would not be tested as rigorously as other vaccines and drugs.
"Will it have been tested as well as other vaccines we put out in the field? No, absolutely not. That would be impossible," she said.
For as long as the clinical tests yielded good results and safety in a small number of people, along in primates, the vaccine may be stamped good to go.
Kieny said the WHO was "engaging with quite a number of developers ... to see what we can do to help accelerate and facilitate the development."
On Thursday, U.S. President Barack Obama rejected the proposal to send the experimental serum given to two American workers to Africa, saying he doesn't feel comfortable yet endorsing it because it still needs further testing.
READ: Ebola Outbreak: Obama Thumbs Down Sending Experimental Serum to Africa
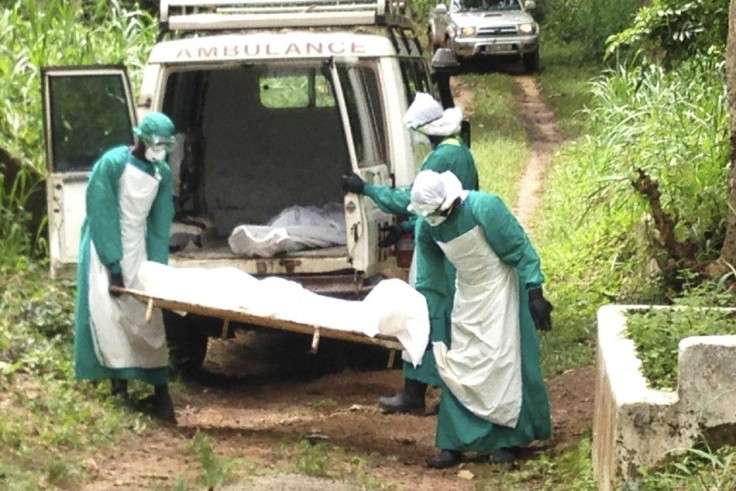
As of Sunday, Keiji Fukuda, UN World Health Organisation assistant director-general for health security, said there have been 1,825 cases reported. The body expects the number of infections to increase.
"What is difficult in this situation is that we are dealing with countries with weak health systems. And we are dealing with areas in which practices like good infection prevention and control practices are not the norm in some of the hospitals and in families and communities," Dr Fukuda said.
The risk of acquiring the Ebola virus is highly preventable through the time-tested soap and water solution.
READ: Ebola Outbreak Preventable Through Proper Hygiene, Washing with Soap & Water; Spanish Priest Confirmed to Have Virus